I enjoyed reading Dr Jeyakumar's letter dated Jan 2021.
He raised many pertinent, relevant issues, and I am sure has been sitting in the back of the minds of many of us. The tone of Dr Jeyakumar's letter contrasts 180 degrees against an earlier letter from four NGOs who are more bent on allegations and rhetorics than a really genuine search for solutions.
I will try and tease some of the major issues which he has raised and offer a perspective which I hope is founded on good science, which in our fraternity is described as evidence-based medicine (EBM).
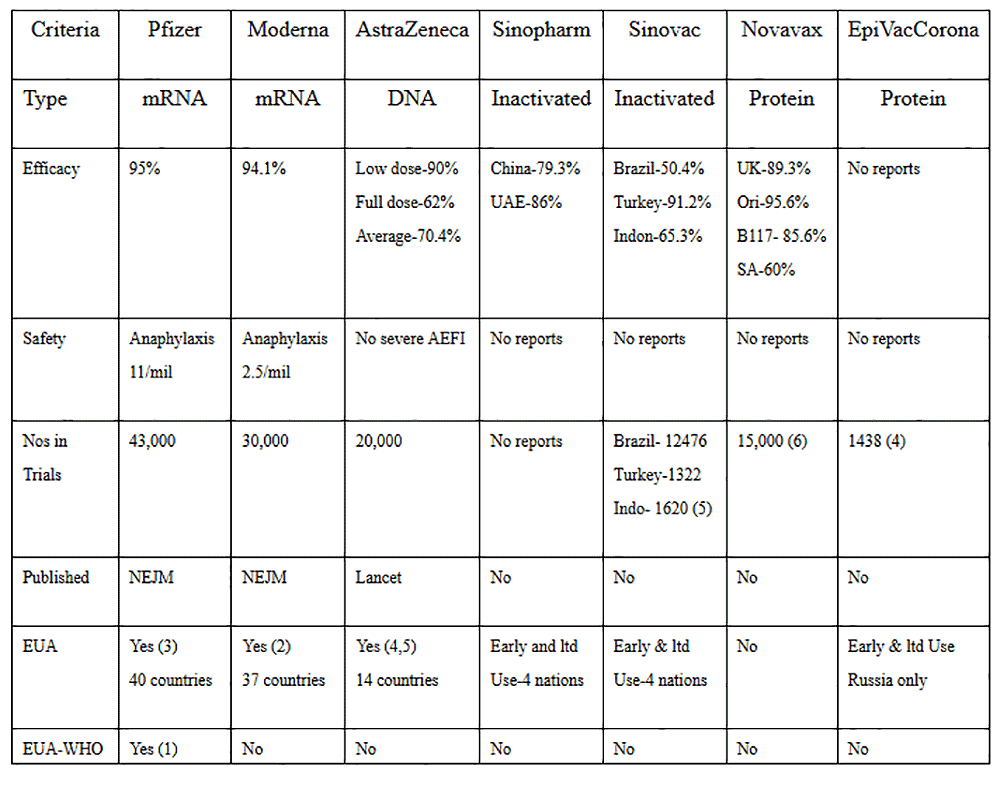
Much of the concerns are directed towards the utilisation of "new" mRNA (Pfizer-BioNTech and Moderna-National Institute of Health) and DNA viral vector technology (AstraZeneca-University of Oxford) vaccines versus the "old" classical live-attenuated, inactivated (Sinovac, Sinopharm, Bharat) or the protein-based vaccines (Novavax, EpiVacCorona-Russian Vector Institute).
Up until today, Jan 29, 2021, there are 102 million global cases of Covid-19, with 2.2 million deaths. In combination with public health measures of masking, distancing, hygiene, 3Ws (wash, wear, warn), avoiding 3Cs (crowded, confined, close) spaces, good ventilation, vaccines are a major tool to exit from this raging pandemic.
I am not sure how much longer you can wait for a vaccine to accelerate this end-game strategy to allow a return to near normalcy. Personally, I think we should not wait a minute longer. There are vaccines at our disposal to accelerate this process. I think the Covid-19 vaccines should have been rolled out in Malaysia like yesterday!
Let us critically study the vaccines that are available. In my opinion, before they are rolled in a mass vaccination program in Malaysia, they must satisfy the following major criteria:
1. Efficacy – The World Health Organisation (WHO) and the US Food and Drug Administration (FDA) have decided that the vaccine must be at least at least 50% efficacy
2. The vaccines must be proven to be safe. This seems to be the primary concern of most people. Vaccine trials have been ongoing since the 1950s. It has been established in the science of vaccinology that more than 90% of adverse effects following immunisations (AEFI) would be unravelled within two months of the vaccine trials. Thus the FDA has mandated that there should not be any Safety Signals for at least two months of the use of the vaccines in the trials.
3. The candidate vaccines must have undergone Phase 3 clinical trials with sufficient numbers in both the treatment and placebo groups to be sufficiently powered to drive significant conclusions on the vaccines' efficacy and safety.
4. The Phase 3 vaccine trials must be able to be validated and verified by the scientific fraternity and must be published in peer-reviewed scientific journals
5. The candidate vaccines must meet the stringent criteria set by the national regulatory bodies and if proven efficacious and safe, will be licensed for Emergency Use Authorization (EUA) by the respective national regulatory agencies
6. The candidate vaccines will be reviewed by the Strategic Advisory Group of Experts (Sage) on vaccines of the WHO for consideration of pre-qualification status for global use.
7. All of the abovementioned criteria is best illustrated and examined in Table 1 below.
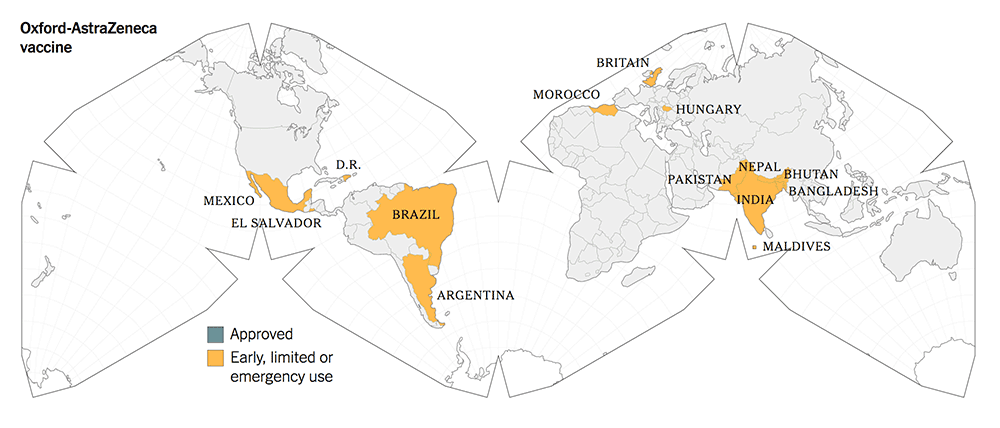
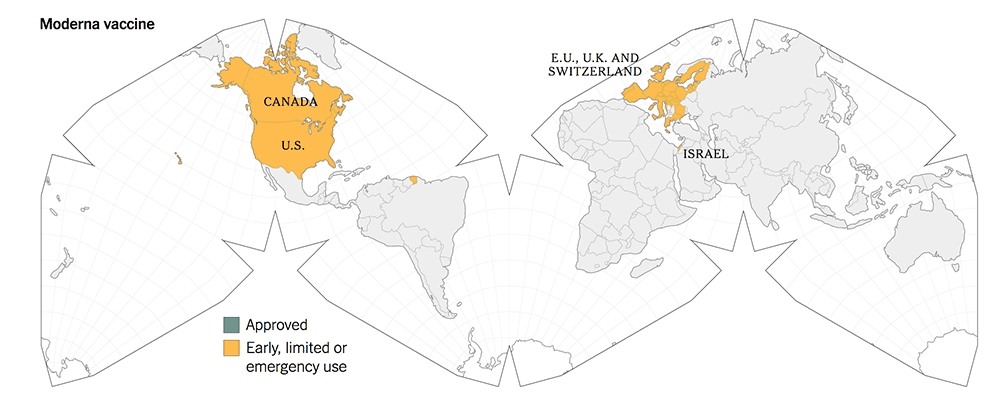
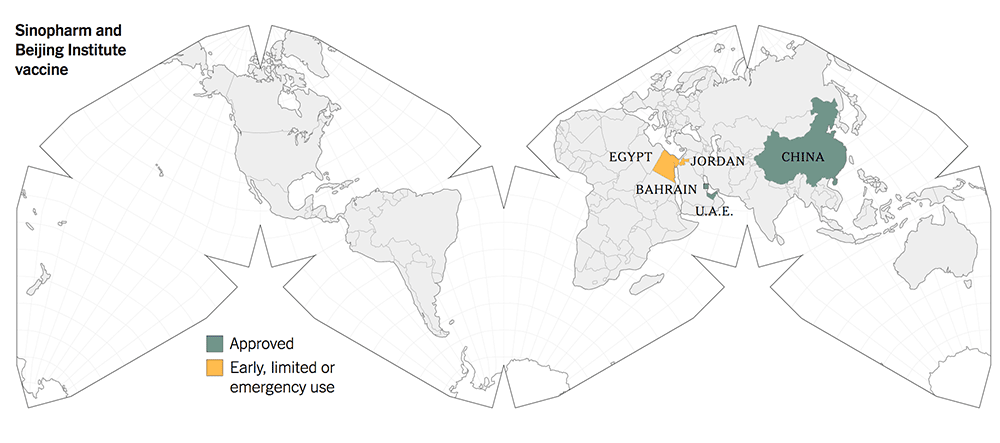
The following maps give a graphic illustration of the approval of the vaccines or early, limited or emergency use in the countries in the world.
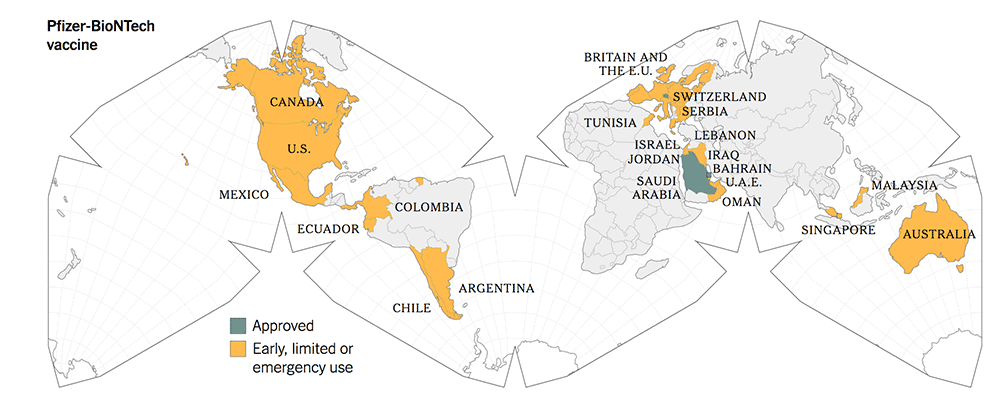
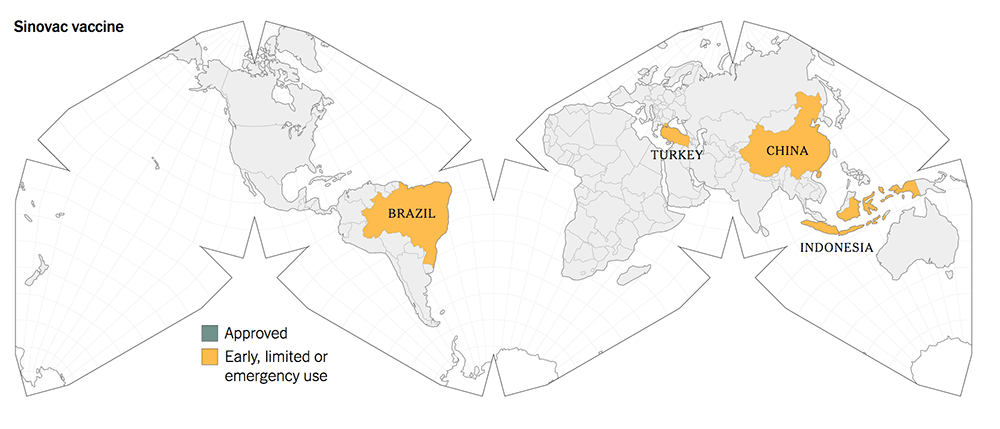
Before you make your decisions, based on the summary Table 1, kindly note the following salient features:
1. Only the new-tech vaccines have been published in scientific journals.
None of the old-tech vaccines has been published scientifically. They have only been revealed in press releases. Thus we are unable to verify the claims of the makers of the old-tech vaccines.
2. The new-tech mRNA and DNA vaccines have superior efficacy when compared to the classic old-tech vaccines. The mRNA vaccines have 95% efficacy rates.
Except for one case in the Pfizer trial, none of the vaccine (5%) recipients developed severe Covid-19 disease. This would have the knock-on effect of preventing the mRNA vaccine recipients from being hospitalised, let alone occupy an ICU or a ventilated bed.
The Israelis have immunised just under 50% of their population. Even prior to the administration of the second dose of the Pfizer vaccine, they have observed a 60% reduction in the hospitalisation rates of their vaccines who are older than 60 years old .
This is a most welcome benefit of the new-tech mRNA vaccines and would contribute immensely towards protecting the capacity of the healthcare facilities and preventing them from being overwhelmed.
3. Except for the very low rates of anaphylaxis in the mRNA vaccines, the new-tech vaccines are safe. There are no published reports of the safety aspects of the old-tech vaccines. This aspect of a vaccine is critical, and one must be fully convinced with sound safety data before even allowing any vaccine to be rolled out.
4. The three new-tech vaccines have about 90,000 volunteers tested in their trials. This compares against 31,856 in the old-tech vaccines trials. Numbers are crucial to scientifically ensure that the trials are sufficiently powered to make sound conclusions on efficacy and safety.
5. All three new-tech vaccines have been licensed for Emergency Use Authorization (EUA) since Dec 2020. They have been approved more widely in more countries.
The old-tech Chinese vaccines from Sinovac and Sinopharm were approved for early and limited use by the Chinese government in June 2020, whilst they were still in Phase 1 / 2 trials.
This is unthinkable and unprecedented according to the scientific fraternity because safety and efficacy had not been fully established then. There should not be any short cuts in the scientific trials.
This would have the effect of releasing to the vaccinated population vaccines which have not been fully studied and may risk unknown safety concerns and may not even be shown to be effective. The move will adversely affect vaccine confidence amongst both the scientists and the general public.
Similarly, the Russian old-tech vaccine EpiVacCorona, was granted early and limited use by Russia in October 2020, while it was still being studied in Phase 1 / 2 trials.
6. Only one vaccine has been granted EUA by the WHO. This is the new-tech Pfizer mRNA vaccine. The other two new-tech vaccines are being considered for EUA by the WHO.
I think I will stop here and allow the readers to decide on which vaccine should be their chosen option. This decision must be based on an informed choice that must not be driven by emotions but by sound EBM.
There is a genuine global shortage of Covid-19 vaccines. All the vaccine manufacturers are scrambling for raw materials and doing their utmost best to ramp up their production numbers.

In my opinion, any Covid-19 vaccine that has been scientifically proven to be safe and efficacious by global and national regulatory bodies should be grabbed and immediately administered.
Any safe and efficacious vaccine is better than no vaccines at all. This is the only other way for the world to exit the pandemic. Thus the Covax initiative is a very honourable and humanitarian gesture because it attempts to provide equitable access to vaccines to all countries irrespective of their GDP status. Their tagline being: "No one is safe until everyone is safe."
I personally feel that Covid-19 immunisation should not be made mandatory. There are various concerns about the vaccines which can only be won over with careful, patient and smart risk communications.
The hard-core anti-vaccine protagonists are far and few in between, but they are very noisy, destructive and irresponsible on social media. We can beat them at their own game.
It is very important for the Health Ministry, Ministry of Religious Affairs, NGOs, professional health organisations and KOLs (key opinion leaders) to step up their game and win over those who are vaccine-hesitant, who probably make up about 30% of our population.
If you still feel wary and unsure of the new-tech mRNA and DNA vaccines, then the option of the classical old-tech vaccine is available, but they should first be licensed by their respective national regulatory bodies, in the case of Malaysia, the National Pharmaceutical Regulatory Agency (NPRA).
The endpoint is to get as many of our eligible rakyat immunised. We are not sure exactly when herd immunity will be achieved. It is probably when about 65-80% of our population have been immunised.
This is obviously a national agenda to immunise the rakyat to achieve the level of herd immunity to confer protection to all others. Therefore, the vaccine should be made readily available, preferably free of charge, and if sold in the private healthcare facilities, it should be easily accessible and affordable.
DR MUSA MOHD NORDIN is a paediatrician and chairperson of the Federation of Islamic Medical Associations advisory council. - Mkini
The views expressed here are those of the author/contributor and do not necessarily represent the views of MMktT.




No comments:
Post a Comment
Note: Only a member of this blog may post a comment.