COVID-19 | Health Minister Khairy Jamaluddin said he has directed the National Pharmaceutical Regulatory Authority (NPRA) to publish weekly reports on adverse events associated with Covid-19 vaccines.
The push towards greater transparency is a bid to instil greater confidence in the vaccines’ safety and encourage more people to take the booster shots.
“I urged the public to please refer to the information published by the NPRA. I have directed the NPRA to publish weekly reports on adverse events following immunisation (AEFI) so the public will know how many AEFI cases, what category of AEFI, and whether the cases circulated on social media are true.
“In most cases, we find that vaccination has nothing to do at all with these cases, which are caused by something else,” he told a press conference in Putrajaya today.
The Health Ministry has been providing regular updates on AEFI cases via its Github data repository since September last year, though a section for certain types of AEFI cases has not been updated since November.
In several recent press conferences, it also provided additional information such as statistics comparing booster doses to earlier doses.
However, Khairy today conceded this was not enough and more needs to be done to win the confidence of those who have yet to receive a booster dose, which is more than 45 percent of Malaysia’s adult population.
Booster AEFI low compared to initial doses
This came amid allegations that booster doses are causing severe adverse reactions, but Khairy countered this by pointing out there are fewer AEFI reports involving booster doses compared to earlier doses.
Up to Jan 31, he said the NPRA has received 24,788 AEFI reports including 1,004 involving booster doses. This corresponds to an overall AEFI reporting rate of 390 per million doses, or 84 per million for booster doses alone.
Of the 1,004 AEFI reports involving booster doses, only 64 are classified as serious. This equates to a reporting rate of 5 per million doses, which is again lower than the overall reporting rate of 27 per million doses.
“It means the AEFI reports for booster doses, by proportion, are far fewer than reports for primary vaccinations. I have to raise this issue because there are so many allegations claiming booster doses are causing serious adverse reactions,” he said.
As for the primary vaccination campaign for adolescents, Khairy said 74 serious AEFI cases have been reported out of 5,656,469 doses administered, meaning a reporting rate of 13 per million doses. All the cases have since recovered.
For children, Khairy said there are no AEFI reports to date but it is still early in the vaccine rollout for children. For the record, children aged five to 12 years receive a reduced-dose version of the Pfizer vaccine that only contains one-third of the dosage received by adolescents and adults.
Adolescents and children are not yet eligible for booster doses.
In a statement released separately, the Health Ministry said the most common AEFI after a booster shot are fever, headaches and stress reactions towards the injection itself.
“Most AEFI reported are mild and usually subside in a few days without treatment,” it said.
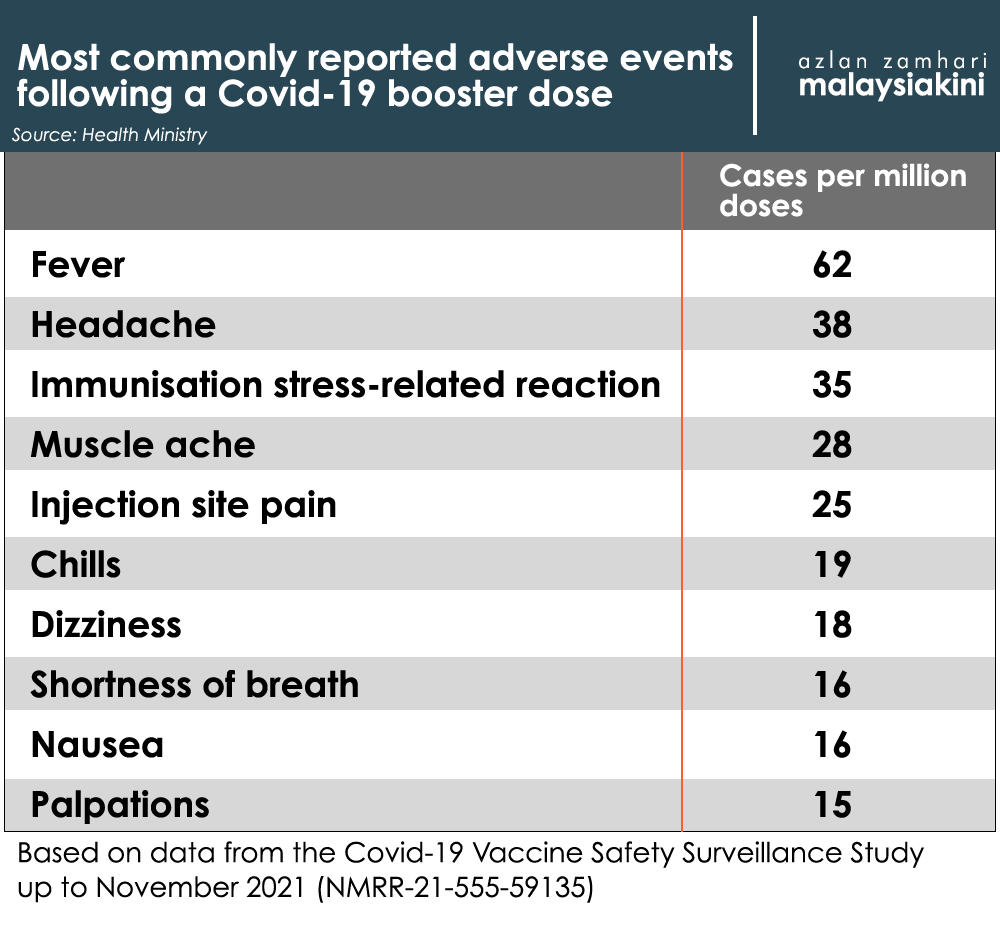
The statement also provided a breakdown of various adverse events according to the vaccine given.
It shows the overall reporting rate for Pfizer’s Comirnaty vaccine to be higher at 503 per million doses, compared to 235 per million for Sinovac’s Coronavac vaccine and 228 for AstraZeneca’s vaccine.
When it comes to serious AEFI cases, however, the reporting rate is similar across the three most used vaccines – 28 per million for Pfizer, 26 per million for Sinovac, and 26 per million for AstraZeneca.
For deaths that occur shortly after immunisation, the reporting rate is 8 per million doses for Pfizer, 12 per million for Sinovac, and 6 per million for AstraZeneca.
The figures are up to Jan 31 based on the AEFI reports received by the NPRA, but the causality of some reported cases have yet to be established.
Meanwhile, the Pfizer vaccine has been linked to 45 cases of pericarditis or myocarditis (inflammations of the heart), which is a known side effect of the vaccine. Forty-three involve the primary vaccination doses while the other two are linked to booster doses.
This equates to a reporting rate of 1.22 reports of pericarditis or myocarditis per million doses.
The AstraZeneca vaccine, meanwhile, is known to cause rare cases of thrombotic thrombocytopenia – a specific type of blood clot.
According to NPRA’s figures, however, only three cases have been reported. This equates to 0.56 cases per million doses.
- Mkini



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.