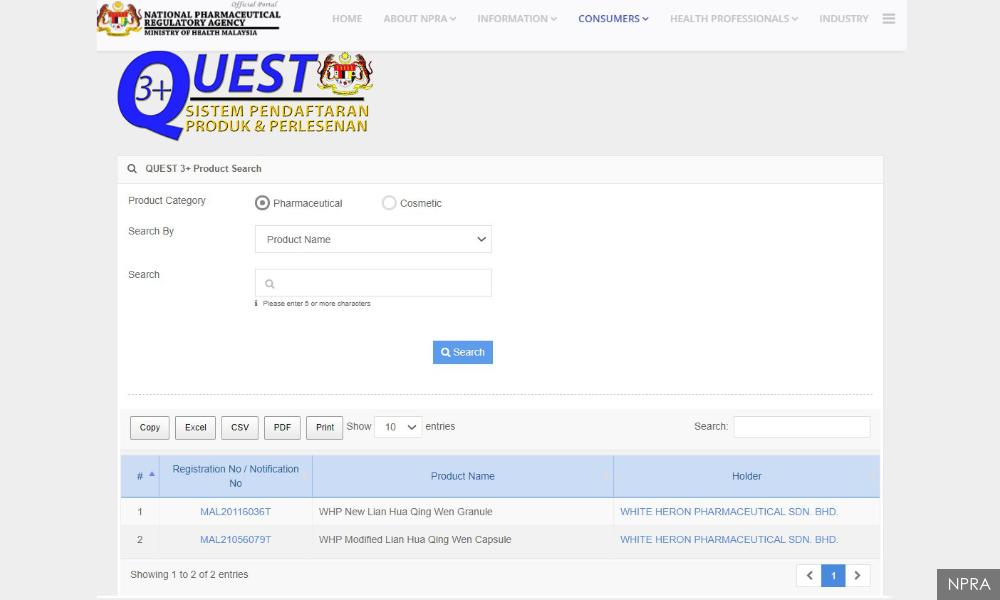
The WHP Modified Lian Hua Qing Wen Capsule, recently licensed by the National Pharmaceuticals Regulatory Agency, was not registered as a Covid-19 treatment, the agency said.
When asked if the tablets were licensed for Covid-19 treatment, NPRA said the tablets were registered as “traditionally used to reduce body heatiness, relieve cough and phlegm”.
The NPRA's move to license these tablets was earlier lauded by Sarawak minister Dr Sim Kui Hian and former minister Teresa Kok as a boon in the fight against Covid-19.
This is because a similarly named traditional Chinese medicine product has been used in China to treat mild to moderate Covid-19 cases.
However, both politicians have deleted their social media postings lauding the move.
This comes after China’s Yiling Pharmaceutical denies manufacturing the tablets licensed in Malaysia.
In a statement yesterday, Yiling Pharmaceutical said it has no relations with White Heron Pharmaceutical Sdn Bhd, which is licensed to sell the Lianhua Qingwen tablets in Malaysia.
“Both products, namely ‘WHP New Lian Hua Qing Wen Capsule’ and ‘WHP New Lian Lua Qing Wen Granule’, have nothing to do with our company.
“We have never had any connection or any form of co-operation with White Heron Pharmaceutical.
“We have never authorised or revealed any information including the ingredients, technology, detection method and indications, etc,” it said.
It added that it holds the exclusive rights to use the name ‘Lian Hua Qing Wen Capsules’ in Malaysia, while its application to license its product for sale in Malaysia is pending.
Before the pandemic, Yiling's Lian Hua Qing Wen Capsule was used in traditional Chinese medicine to treat influenza-like symptoms.
Health director-general Dr Noor Hisham Abdullah and Health Minister Dr Adham Baba did not respond to requests for comments on the matter.
Malaysian pills not identical to China product
Further checks with NPRA also found that the tablets sold by White Heron Pharmaceutical are not identical to those manufactured by Yiling Pharmaceutical.
One key missing ingredient in the White Heron Pharmaceutical product is ephedra sinica, better known as ma huang, the NPRA confirmed in an email to Malaysiakini.
Although not licensed in Malaysia, the China product is easily available through unlicensed online sellers.
A check with one online seller found that the products were imported by China herbs wholesaler based in Malaysia, and are not registered with NPRA. A box of 24 tablets generally retails at RM24 online.
Last October, the Selangor Health Department seized 1,200 boxes of the China-imported product from an unlicensed seller, who claimed it was a Covid-19 remedy.
Under the Sale of Drugs Act 1952, those convicted of distributing unregistered pharmaceutical products could be fined up to RM25,000 or jailed up to three years, or both.
No significant impact on viral assay
In April 2020, the National Medical Products Administration of China allowed Yiling Pharmaceuticals to state that its product can be used for treating mild to normal cases.
Universiti Tunku Abdul Rahman Department of Chinese Medicine head Te Hian Kong earlier told Sin Chew newspaper that Yiling's product is recommended in China for Covid-19 patients who are experiencing fatigue and fever, and should not be consumed as a prevention.
Te also urged those seeking to use the tablets to consult a traditional Chinese medicine practitioner first as self-administering could trigger other symptoms.
A paper published in the peer-reviewed Phytomedicine journal last month concluded that the Yiling manufactured tablets "could be considered to ameliorate clinical symptoms of Covid-19".
However, the trial involving 284 Covid-19 patients found the tablets did not have a significant impact on the rate of conversion from mild to severe infection.
There were also no significant differences in the viral assay findings, between the group who consumed the tablet and the group who did not, it said. - Mkini




No comments:
Post a Comment
Note: Only a member of this blog may post a comment.