A Health Ministry (MOH) study has confirmed there is no benefit in repurposing the antiparasitic drug ivermectin to treat Covid-19, in line with high-quality studies published on the matter.
Instead, study participants who received ivermectin were three times more likely to report side effects compared to participants who didn’t – unusually cases of diarrhoea.
“Based on the outcomes of the Ivermectin Treatment Efficacy in Covid-19 High-Risk Patients (i-Tech) study, ivermectin cannot be recommended for inclusion in Covid-19 treatment guidelines as ivermectin does not reduce risk of severe illness from Covid-19.
“The MOH continues with prior advice that ivermectin only be used within clinical trial settings with monitoring,” its director-general Dr Noor Hisham Abdullah said in a statement today.
The recommendation is in line with those from the World Health Organisation and many other health authorities around the world.
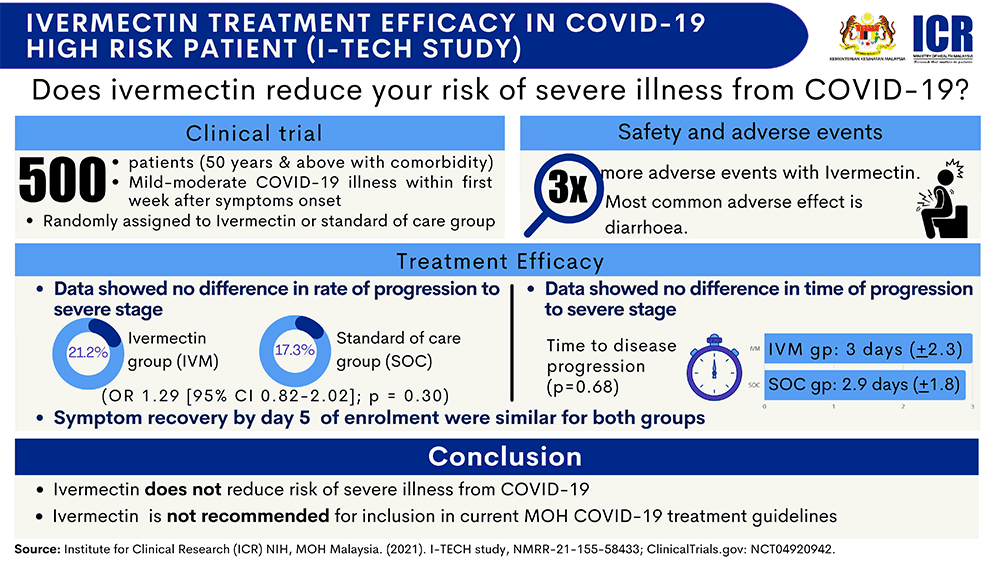
500 Covid-19 patients took part in i-Tech study
Noor Hisham said the i-Tech study had enrolled 500 Covid-19 patients above 50 years old with at least one comorbidity, who had been admitted to hospital with either Stage 2 or Stage 3 Covid-19.
Stage 2 refers to those with mild symptoms, while Stage 3 refers to those who have pneumonia but do not require supplementary oxygen. Meanwhile, Stage 4 patients have pneumonia requiring supplementary oxygen, while Stage 5 patients will require ICU admission and possibly mechanical ventilation.
The study was conducted in 20 government hospitals as well as the Maeps Quarantine and Covid-19 Treatment Centre in Serdang, Selangor, by experts from the Institute for Clinical Research (ICR) and the National Institute of Health (NIH).
The participants were randomly assigned to either receive the ministry’s standard Covid-19 treatment protocol, or the same protocol with the addition of a five-day course of ivermectin.
The dosage administered was 0.4mg of ivermectin per day for each kilogram the participant weighs. For example, a person weighing 65kg would receive 26mg of ivermectin per day.
Among others, they were assessed based on whether they progressed to severe disease (Category 4 or 5), how long it takes to progress to severe disease, whether the symptoms resolved at the participants’ fifth day in the trial, and whether they were still alive after 28 days.
Four participants were ultimately excluded from the study for not meeting the study criteria, while another six withdrew from the study after expressing concerns about ivermectin’s side effects.
Of the participants who remained in the study, Noor Hisham said ICR director Dr Kalaiarasu M Peariasamy reported that 21.2 percent of those who received ivermectin progressed to severe Covid-19, compared to 17.3 percent in the group who received standard care.
The rate of progression is also similar in both groups, taking 3 days in the ivermectin group and 2.9 days in the standard care group.
For both measures, the difference between the ivermectin group and the standard care group were considered small and not statistically significant.

Likewise, Noor Hisham said the study’s principal investigator Dr Steven Lim Chee Loon found that ivermectin made no clear difference in terms of ICU admission rate, usage of mechanical ventilators, symptom resolution, blood test parameters, and chest x-ray results.
The odds of fully recovering from Covid-19 symptoms within five days of treatment is also similar for both groups.
The ivermectin group did have a slightly reduced mortality rate on the 28th day after treatment began, but the finding was not statistically significant.
“According to Lai Nai Ming and Karuthan Chinna from Taylor’s School of Medicine who provided independent statistical analysis, the i-Tech study cannot confirm whether ivermectin administered in hospital compared with standard of care alone leads to fewer deaths at 28 days.
“This is due to the small number of deaths (13 deaths out of 490 participants) which provided a limited evaluation of the result,” said Noor Hisham.
He noted that the findings of the i-Tech study were in line with other large studies, namely the Ivercor-Covid19 study in Argentina and the Together study in Brazil, both of which did not support the use of the drug for Covid-19 treatment.
'Medical practitioners should not endorse ivermectin'
Moving forward, Noor Hisham said the research team plans to publish the study in peer-reviewed journals to inform future ivermectin studies including meta-analyses.
“It is hoped that the findings of this local study will inform medical practitioners in Malaysia and the public who often asked about ivermectin’s efficacy in the clinical treatment of Covid-19.
“Medical practitioners are reminded not to endorse the use of ivermectin including to advertise it or to sell it illegally for Covid-19 treatment until there is stronger scientific evidence (of its efficacy),” he added.
Ivermectin is an anti-parasitic drug that is normally used for treating parasitic diseases such as head lice, scabies, and river blindness. However, it is only approved for animal use in Malaysia.
Nevertheless, various groups and even MPs have touted it as a cheap and effective treatment for Covid-19 based on a mix of misinformation and poor-conducted studies.
At least one major paper claiming to show the benefits of using ivermectin to treat Covid-19 has since been retracted after further analysis purportedly found irregularities that pointed to scientific fraud.
Since then, higher-quality studies have been conducted, which were largely either inconclusive or found no benefit in using ivermectin to treat Covid-19.
When researchers at the Cochrane Collaboration performed an exhaustive review of the available data on the issue up to May 26, they reported: “Based on the current very-low‐to-low‐certainty evidence, we are uncertain about the efficacy and safety of ivermectin used to treat people with Covid‐19 in the inpatient and outpatient settings and to prevent a SARS‐CoV‐2 infection in people after having high‐risk exposure.
“There is also no evidence available from the study pool as to which is the best dose and regimen of ivermectin.
“Overall, the reliable evidence available does not support the use of ivermectin for treatment or prevention of Covid‐19 outside of well‐designed randomised controlled trials.”
The review includes a mechanism for assessing studies for risk of bias that can result from flaws in designing or performing the studies. - Mkini




No comments:
Post a Comment
Note: Only a member of this blog may post a comment.