The Health Ministry will open four large vaccination centres (PPV) in the Klang Valley in a bid to speed up the Covid-19 booster shot rollout as the Omicron variant threat becomes imminent.
The vaccination centres at World Trade Centre in Kuala Lumpur, Axiata Arena in Bukit Jalil, Ideal Convention Centre in Shah Alam, and Soka Gakkai Hall in Klang will begin operations on Jan 15 (Saturday).
“We will look into the necessity (of integrated PPVs) in other major cities as well, and if there is a need, we may also open integrated PPVs in other major cities,” said Health Minister Khairy Jamaluddin at a press conference in Kuala Lumpur today.
He said booster dose appointments will continue to be issued through MySejahtera and SMS notifications. Those who want to join a waiting list can still contact local clinics listed on ProtectHealth’s website to do so.
The government is aiming to ramp up the booster roll-out from 120,000 doses per day last month to 200,000 doses per day. It expects most people eligible for a booster shot to get receive the vaccine by the end of February.
Khairy said 30 percent of the Malaysian adult population have received their booster shots, including 52 percent of the elderly population.
AEFI report steady amid booster roll-out
As for reports of adverse events after immunisation (AEFI), the minister said there is no increase in the reporting rate and trends so far in the booster rollout, compared to the earlier primary vaccination campaign.
AEFI refers to any adverse event that occurs after immunisation, regardless of whether it is related to the vaccine or not. This is in contrast to an “adverse reaction” where a causal link between the vaccine and the adverse event is suspected or established.
An AEFI is considered serious if it leads to hospitalisation, disability, or death, or otherwise life-threatening.
Most drug regulators, including Malaysia’s National Pharmaceutical Regulatory Agency (NPRA), have systems in place to receive and investigate AEFI reports from doctors and members of the public, including for Covid-19 vaccines.
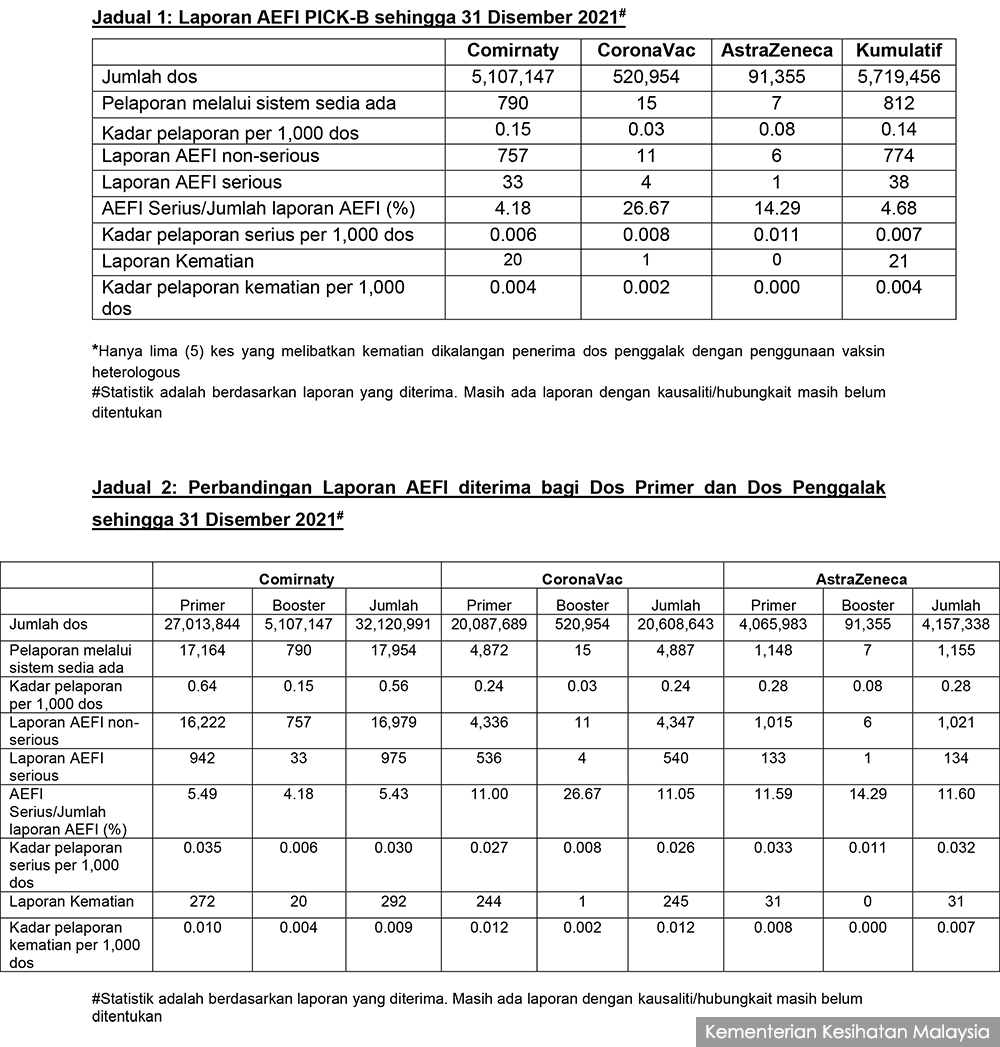
Khairy said the NPRA has received 24,042 AEFI reports in relation to Covid-19 vaccines including 812 for booster shots, out of 57,119,777 vaccine doses given up to Dec 31 last year of which 5,719,456 are booster shots.
This corresponds to an overall reporting rate of 0.42 per 1,000 doses, or 0.14 per 1,000 doses for booster doses alone.
“Fever, injection site pain, headache, and fatigue are the most commonly reported adverse events reported by vaccine recipients […]
“Of the serious cases, all of them have recovered. These are cases of allergies and so on, but there are no serious cases that require follow-up treatment,” he said.
As for reports of serious AEFI associated with booster doses, there are only 38 reports received so far, or 0.007 reports per 1,000 doses.
A breakdown of the AEFI reports by vaccine type was also provided after the press conference.
It shows the reporting rate of serious AEFI are 0.006 per 1,000 doses for Pfizer boosters, 0.008 for Sinovac boosters, and 0.011 per 1,000 doses for AstraZeneca doses. These reporting rates are lower than those for the primary vaccination.
There are also 20 reports of death associated with the Pfizer booster and one associated with the Sinovac booster, corresponding to a reporting rate of 0.004 per 1,000 doses and 0.002 per 1,000 doses respectively. This includes five cases involving heterologous vaccination.
However, the figures came with a caveat noting: “Statistics are based on reports received. There are still some reports where causality/association has yet to be ascertained”.
When asked about two cases of AEFI deaths reported in Kajang Prison, Health Ministry director-general Dr Noor Hisham Abdullah - who is also present at the press conference - said investigations are still ongoing. - Mkini




No comments:
Post a Comment
Note: Only a member of this blog may post a comment.